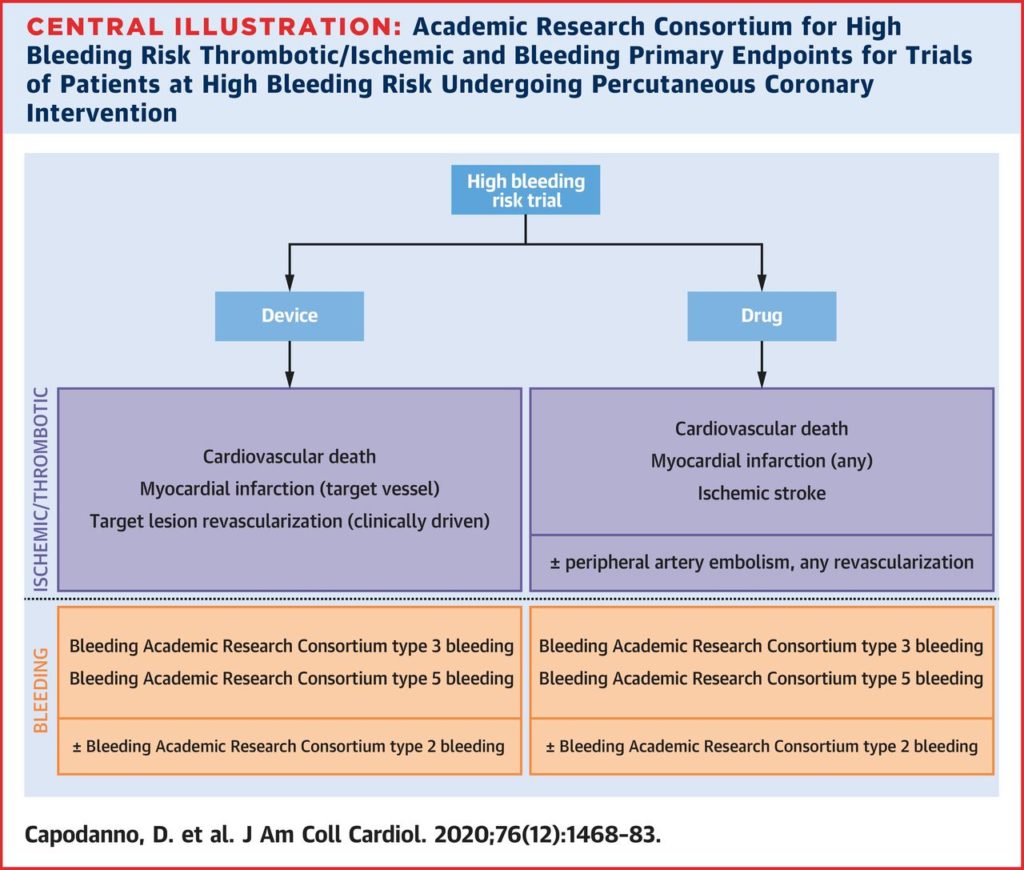
“Trial Design Principles for Patients at High Bleeding Risk Undergoing PCI” : The second ARC HBR document has just been published last week !
Co-written by Davide Capodanno, Marie-Claude Morice, Dominick J. Angiolillo, Deepak L. Bhatt, Robert A. Byrne, Roisin Colleran, Thomas Cuisset, Donald Cutlip, Pedro Eerdmans, John Eikelboom, Andrew Farb, C. Michael Gibson, John Gregson, Michael Haude, Stefan K. James, Hyo-Soo Kim, Takeshi Kimura, Akihide Konishi, Martin B. Leon, P.F. Adrian Magee, Yoshiaki Mitsutake, Darren Mylotte, Stuart J. Pocock, Sunil V. Rao, Ernest Spitzer, Norman Stockbridge, Marco Valgimigli, Olivier Varenne, Ute Windhovel, Mitchel W. Krucoff, Philip Urban and Roxana Mehran
Abstract:
“Investigating the balance of risk for thrombotic and bleeding events after percutaneous coronary
intervention (PCI) is especially relevant for patients at high bleeding risk (HBR). The Academic Research Consortium for HBR recently proposed a consensus definition in an effort to standardize the patient population included in HBR trials. The aim of this consensus-based document, the second initiative from
the Academic Research Consortium for HBR, is to propose recommendations to guide the design of clinical trials of devices and drugs in HBR patients undergoing PCI. The authors discuss the designs of trials in HBR patients undergoing PCI and various aspects of trial design specific to HBR patients, including target populations, intervention and control groups, primary and secondary outcomes, and timing of endpoint reporting.”
